INTRODUCTION
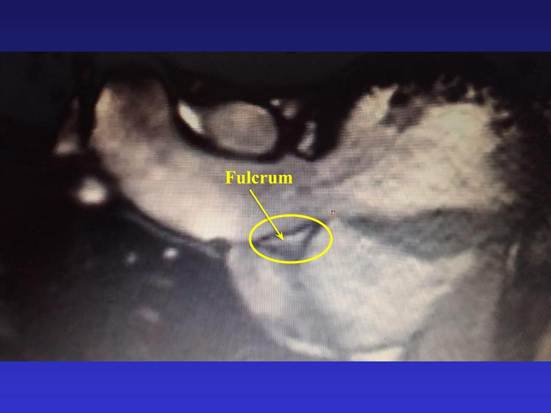
The contraction and distension of the continuous myocardium fibers, whose ends must be attached to a "fixed point" to have a mechanical effect, would not be effective without the existence of the cardiac fulcrum. This structure is found at the beginning and end of the myocardium, ensuring the functionality of the cardiac system, (1) and allowing the forces to be adequately distributed, not only to support but also to stabilize the sequential and asymmetrical cardiac movements. To comply with this mechanism of being subjected to traction in a magnitude of about one hundred thousand cardiac cycles per day, the fulcrum must meet certain conditions: stability, resistance, elasticity, and plasticity. As it is subjected to loads, these faculties allow the fulcrum to reach a certain level of stress, modify its spatial location with the variation of these loads, and then recover when the loads are removed (Figure 1).
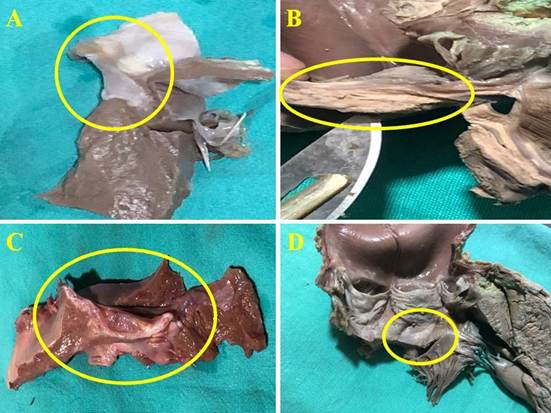
Figure 1
Cardiac fulcrum (yellow circle) in porcine (A), adult human (B and D), and bovine (C) hearts.
Cardiac mechanics is complex, because it must integrate its ejection, suction and filling properties under different successive and concatenated phases through the continuous helical myocardium. (2) The function of cardiac fibers is described as a homogeneous and synchronous faculty, and little attention is paid to the disposition adopted in the helical construction and the sequence of contraction. It is impossible to interpret cardiac function without taking into account its morphology and activation circuit with the corresponding sequential movements. (3) Cardiac activation is a consequence of the propagation of stimuli through its helical muscular structure. (4) The mechanism of suction and ejection requires a structure-function integration that interprets various aspects of its dynamics consistent with the propagation of excitation. (4,5)
It was classically considered that both mechanical contraction and electrical activation of the heart were homogeneous processes. Thus, contraction would occur "en bloc" during systole and relaxation would occur homogeneously during diastole. Thus, systole would be synonymous with cardiac contraction and diastole with relaxation. At this stage of knowledge, more complex mechanisms should be considered. (5) Although various aspects of electrical stimulus propagation in the heart have long been known, the advent of three-dimensional navigators and electroanatomical mapping have allowed more detailed studies of myocardial activation. It was thus evidenced that endocardial activation "occupies" approximately 60% of the initial surface QRS. The "rest" of the QRS corresponds to myocardial and epicardial activation. The beginning of ventricular activation evidenced by the QRS is then exclusively endocardial; during the intermediate phase both activations coexist and the end, during suction, in the so-called protodiastolic phase of myocardial contraction (PPMC) of both ventricles, there is an active process whose activation is exclusively myo-epicardial. (4)
In this study, the structural properties of the cardiac fulcrum were analyzed in relation to the movements of the myocardial segments during the cardiac cycle. Considering the sequential movements of the myocardium, the cardiac fulcrum is subjected to a functional requirement that implies its role as support and stabilizer of the myocardium, as well as being modeled in its morphology by the stresses occurring in its structure.
METHODS
Thirty-five bovine, porcine and human hearts from the slaughterhouses and the morgue were used in this research: a) 18 two-year-old bovine hearts weighing 800-1000 g; b) 16 human hearts (two from 16 and 23-week gestation embryos; four from 30- and 36-day and 10- and 27-week infants; one from 4-year-old child; one heart from a 10-year-old child weighing 116 g and eight adult hearts with an average weight of 300 g); and c) one porcine heart (400 g). Histology was performed with hematoxylin-eosin (H&E) and Masson's trichrome staining techniques and four-micron sections. Formalin 10% was used as buffer. Immunostaining (s100-neurofilaments) was also performed.
The single continuous and helical myocardium was deployed according to a previously published technique. (6) The conjunction of the origin and end of the continuous myocardium in a support that we call cardiac fulcrum constitutes a meeting point that allows the heart to adopt the spatial arrangement of a set of fibers twisted unto themselves, like a laterally flattened rope forming a double helix that defines the two ventricular chambers. This research on the properties of the cardiac fulcrum complements previous findings on the myocardium, the cardiac fulcrum and ventricular activation studies with the Carto system. (2,4,6,7).
RESULTS
The microscopic analysis of the bovine cardiac fulcrum shows a trabecular osteochondral matrix with segmental lines. Its general structure resembles the metaphyseal growth of long bones. The same histological findings have been found in the chimpanzee, buffalo, sheep, goat, antelope, deer, giraffe, camel, dog, cat, pig, sea lion, horse and elephant fulcrum. Until our research, no function or sense of its presence was ever assigned to it, nor was it described in humans (8).
The histology of the fulcrum in adult humans (approximately 25 mm long and 15 mm wide) has shown a chondroid-tendinous matrix, which needs further clarification. In principle, there is similar consistency in the detection, location and morphology of the fulcrum in all the hearts analyzed. They present myocardial insertion in the fulcrum, integrating a cardiomyocyte-matrix unit, independently of the osseous, cartilaginous or tendinous nature of the fulcrum in the different specimens. This difference corresponds to the higher power developed in bovids, which makes it necessary to have a more rigid support. This point of fixation implies that, as in all muscle, it acts as a support and also as a bearing, preventing the ventricular rotation force, either by torque (twisting force) or torsional effort, from extending to the great vessels, thus dissipating the energy produced by the movement of the muscular helix. In all the hearts, the myocardium was found to be tethered to the fulcrum, which we can symbolize as "the ivy to the stone", integrating a cardiomyocyte-matrix unit, either osseous, cartilaginous or tendinous.
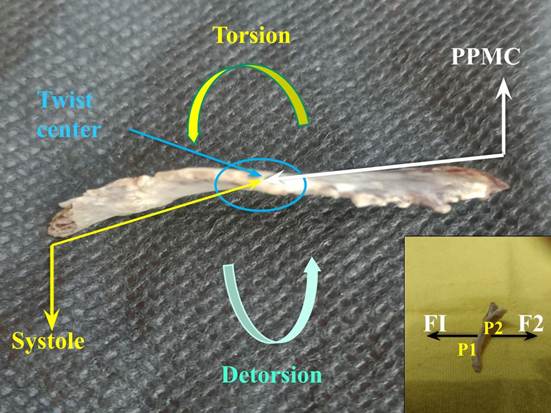
If a force is applied at one end of the fulcrum, this is displaced, since it is a semi-floating structure, but if a force F2, equal and opposite to F1, is applied at the insertion point P2, at the end opposite to point 1 (P1), as can be seen in the Figure 2 inset, the fulcrum is not destabilized. As both forces are produced by the same myocardial muscle fiber, which originates at P1, and ends at P2, the action and reaction effects compensate for the displacements. The same can be applied to the totality of myocardial muscle fibers along the lines of insertion. The forces in the myocardial muscle fibers compensate each other and with the reactions in the fulcrum, which is elastic enough to bear them.
Figure 2
The yellow arrows show the orientation of the cardiac fulcrum movements during systole; the black arrows during suction (protodiastolic phase of myocardial contraction -PPMC). The inset shows that if only one force is applied to the fulcrum, it would be displaced; on the contrary, if a force F2 equal and opposite to F1 is applied at the insertion point P2, opposite end to P1, the fulcrum does not destabilize.
The histological conformation of the bovine fulcrum shows a less dense area in its center (marrow) in relation to the cortical area (Figures 3, I and II). These characteristics, compared with the human and porcine fulcrum (Figures 3, III and IV), explain what is shown in relation to the body weight and the absolutely osseous characteristics in the former and cartilaginous in the rest, associated to the power developed. In analogy, the bovine fulcrum has defined cortical and marrow areas in order to absorb much higher tensions due to its weight, compared with mammals such as man and swine, which present a greater homogeneity in the cartilaginous structure. These differences with the bovine fulcrum do not prevent them from dissipating the powers generated by the myocardium, contributing to the stability and organization of the heart.
Figure 3.
I. Cardiac fulcrum in the bovine heart. The cortical and marrow areas of the fulcrum are well defined. A: The insertion of the myocardial fibers in the chondroid tissue is shown. B: H&E at high magnification (40x) showing an osseous trabecula with osteoblasts and segmental lines. The structure forms the scaffold of trabecular osseous tissue similar to the metaphyseal growth zones of long bones. Osseous trabeculae with osteoblasts and segmental lines secondary to bone apposition are visualized.
II. Bovine fulcrum, cortical and marrow areas. Partially calcified osteochondral tissue is observed in the cortex and osteochondral trabeculae in the marrow that results in a spongy structure with elements of bone marrow in the space. 1: myocardium. 2: fulcrum cortex. 3: fulcrum marrow.
III. Porcine fulcrum: 25x, Masson's staining. 1: porcine cartilaginous fulcrum. 2: peri-fulcrum fibrous tissue. 3: septum. 4: intermingled conduction cardiomyocytes, nerves, and ganglia reaching the fulcrum.
IV. Central area of a human heart fulcrum. Fibrous tendon and cardiomyocytes, H&E (25x).
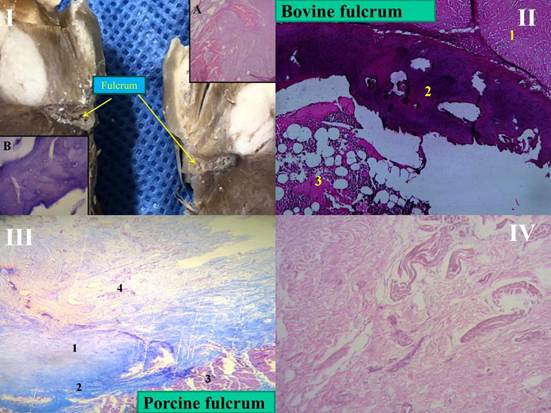
H&E: hematoxylin-eosin staining.
The system of forces to which the fulcrum is subjected is compensated with respect to a central point, but the fulcrum does not remain static in the thoracic cavity but shows small movements due to the different compensations of these forces. For an observer located at the center of the fulcrum all the forces of the cardiac muscle are compensated, but for an external observer the compensation involves fulcrum movement.
As the fulcrum is subject to the forces of the myocardial segments attached to its structure, it obviously registers tensions that even lead to modeling its morphology. This concept is explained by the fact that myocardial movements are sequential and superimposed in the myocardial segments, (9) producing asymmetric stresses that act with their epicenter in the fulcrum. The myocardium inevitably needs this support in order not to destabilize its structure in the face of the unequal movements generated by its forces.
Cardiac activation through the myocardium (Figure 4), whose segments are helically arranged and therefore superimposed, determines sequential stimulation. The contraction is not global or homogeneous but by sectors. Therefore, the contraction, during the 400 ms of the cardiac cycle (systole and suction), is paced. In the face of this asymmetric muscular movement, the fulcrum plays a supporting role so that the heart can have the necessary power to eject and draw out the blood volume. In the face of these displacements generated by the anisotropic activation of the myocardial fibers, the fulcrum acts as a stabilizer since the continuous myocardium originates and ends in this structure. In this way it avoids an accentuation of the displacements so that the cardiac structural scaffolding is not lost. (10)
The orientation of the continuous myocardium fibers and their activation implies a concatenation of muscular movements in cardiac mechanics. These follow one another, giving rise to three active phases according to the segments stimulated: narrowing (right and left segments), shortening-torsion (descending and ascending segments), lengthening-detorsion (cubic segment).
DISCUSSION
During systole, the different segments of the continuous myocardium contract sequentially. Activation begins in the right segment, tethered to the right and anterior sector of the cardiac fulcrum, with continuation in the left, descending and ascending segments. The fundamental peculiarity of this activation is that although at the beginning it is unidirectional, upon reaching the junction of the descending and ascending bands, simultaneity is produced -by transversal activation- in both bands, generating a helical movement essential for the myocardium to expel the ventricular contents at a speed of 200 cm/s (Figure 4 ).
Figure 4
Chord model. Activation sequence (A-F) in the continuous myocardium according to our investigation. The propagation times are observed. The 25.8 milliseconds in B represent the delay of the stimulation to pass from the descending band in A to the ascending band in B (detailed in the black circle).
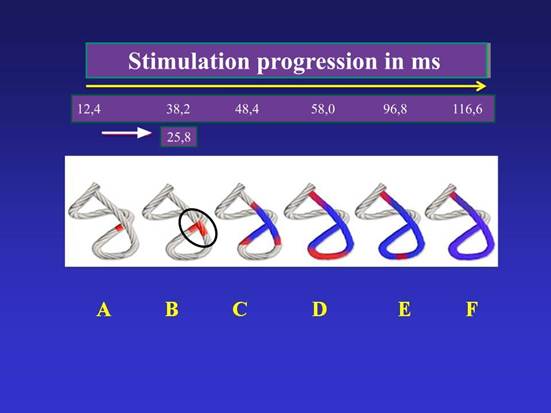
In red: depolarization; in blue: zones already activated.
At the beginning of myocardial activation in the right segment (free wall of the right ventricle), the right end of the fulcrum (where the right segment inserts) moves downward due to the predominantly downward orientation of its fibers. Conversely, the opposite end, where the ascending segment ends, undergoes an upward displacement as it is still relaxed (Figure 2).
The continuation of activation by the left segment (mitral orifice) determines, together with the right segment, the narrowing phase. The right and left segments constitute the basal loop. This contraction defines an external sheath within which the apical loop (descending and ascending segments) will contract. Actually, the right ventricular crescent-shaped free wall is ad latere to the rest of the ventricular mass (septum and left ventricle), since the left segment constitutes part of the posterior epicardial wall of the left ventricle in its superior portion, while the rest of the left ventricular wall is covered externally by the ascending segment.
In this sheath, stimulation runs from the subepicardium to the subendocardium. It then runs through the descending segment and, in our investigation, at an average of 25.8 ms after activation in this segment, the ascending segment contracts (at an average of 38.2 ms from the start of myocardial activation) (Figure 4). This probably occurs because the subendocardial fibers of the descending segment, on the anterior aspect of the left ventricle, pass deep into the mesocardium crossing obliquely with those of the ascending segment, thus facilitating transverse stimulation between the two segments.
At this moment of systolic activation, longitudinal shortening of the myocardium occurs with circular narrowing and the torsion that characterizes its helical function, which implies that the fulcrum undergoes a downward displacement, accompanied by a torque from its right end to the opposite end due to torsion (Figure 2).
When the ejective period ends, the terminal part of the ascending segment remains in the active process of contraction, that is, in its attachment to the cardiac fulcrum, which occurs fundamentally in the antero-inferior portion and posterior face of the latter. This phase occupies about 80-100 ms and is intermediate between systole and diastole. We have called it the protodiastolic phase of myocardial contraction (PPMC) and is the cause of the process of generating negative intracavitary ventricular pressure, resulting in the opening of the atrioventricular valves and the precipitous entry of blood into the ventricles by a suction mechanism. During this phase the myocardium lengthens, narrows and detorsions. (11) Under these tensions the fulcrum at its left end undergoes an upward displacement and generates a torque opposite to the one it had during systole, due to myocardial detorsion. Obviously, this continuous torque in coupling models the fulcrum with a torsion that is well observed in a profile view. The torque is a demonstration of the opposing and unaligned forces that stress the fulcrum; thus, the continuous myocardial ascent and descent together with the torsion-detorsion effect shape its morphology.
The section of the fulcrum is not axisymmetric (symmetrical in relation to its axis); therefore, the torsion-detorsion it undergoes in each cardiac cycle determines deformations in its structure. Thus, the torque (Figure 2) applied at each of its ends during the cardiac cycle causes the free ends of the bar to rotate at an angle Φ, which is called the twist angle. In this deformation the maximum shear strain is produced in the middle of the fulcrum faces and around the axis in which it rotates, thus producing a rotation effect, without achieving an overt translation. In diástole, the fulcrum returns to its position (Figure 5).
Could the myocardium function without the cardiac fulcrum? With its helical configuration, but without the fulcrum, the myocardium would be a Moebius band modified so that the beginning and end of the same fiber would be closed. The closed fibers would determine that their stresses are established as in an elastic band, but without an anchor point or support. The non-existence of the anchor point would not allow the adequate sliding of the fibers between them, losing the correct sequence of their efforts. Physically, we would speak of free and unfixed vectors, losing the correct sequence of their efforts, and the myocardium itself would be exposed to undesired displacements, due to asynchronous movements.
The existence of the fulcrum is inevitable. Also, it has been proven in published research that the fibers are lubricated with hyaluronic acid in order to reduce the friction between them and the loss of energy, so that most of it is transformed into motion. (12)
The upper edge of the fulcrum has a ledge that adapts to the aortic annulus, providing evidence of the pattern of organization of the helical heart. The cardiac fulcrum, in order to maintain stabilization of the myocardium, which originates and ends in its structure, is subjected to sequential tractions by the cardiac movements. These forces exerted on its structure must have practically zero resultant to maintain the equilibrium of the cardiac system, which with its movements generates tensions that are absorbed by the fulcrum, preventing them from being transferred to the aorta. In this way, it avoids the aorta from traction and rotation, which would produce resistance to ejection.
In addition to the contractions that generate myocardial shortening and lengthening, the cardiac fulcrum is subjected to clockwise and counterclockwise rotational movements in its posterior and anterior regions respectively, given the helical constitution of the cardiac fibers and the consequent myocardial torsion-detorsion. The torque force rotates the cardiac descending and ascending segments in opposite directions until their forces are equalized at point 0 (center or twist). In this interplay the fulcrum is modeled around the axis of rotation in which it is located, with its maximum shear strain being produced in the middle of its faces and around the axis of rotation.
CONCLUSIONS
The fulcrum constitutes the support for the myocardium to exert the necessary power, but due to the sequential cardiac movements, it acts as a stabilizer of the whole heart, absorbing the alternating ascending, descending and torsional tractions. This interplay implies not only a limit to the movements of the heart, but also that the fulcrum itself is shaped in its form by these movements through the torque to which it is subjected. Its properties of stability, resistance, elasticity and plasticity allow the myocardium to fulfill its function.
Conflicts of interest
None declared. (See authors' conflict of interests forms on the web).